
At room temperature, PbS is sensitive to radiation at wavelengths between approximately 1 and 2.5 μm. Measuring the resistance change is the more commonly used method. A PbS element can be used to measure radiation in either of two ways: by measuring the tiny photocurrent the photons cause when they hit the PbS material, or by measuring the change in the material's electrical resistance that the photons cause. As an infrared sensor, PbS directly detects light, as opposed to thermal detectors, which respond to a change in detector element temperature caused by the radiation. PbS was one of the first materials used for electrical diodes that could detect electromagnetic radiation, including infrared light. World War II German PbS infrared detector In 2009, PbS nanoparticles have been examined for use in solar cells. Traditionally, such materials are produced by combining lead salts with a variety of sulfide sources.
Lead sulfide-containing nanoparticle and quantum dots have been well studied. The sulfur dioxide is converted to sulfuric acid. Idealized equations for these two steps are: 2 PbS + 3 O 2 → 2 PbO + 2 SO 2 PbO + C → Pb + CO A major process involves smelting of PbS followed by reduction of the resulting oxide. Since PbS is the main ore of lead, much effort has focused on its conversion. Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors. In fact, lead sulfide was one of the earliest materials to be used as a semiconductor. Like the related materials PbSe and PbTe, PbS is a semiconductor.
The presence of hydrogen sulfide or sulfide ions may be tested using "lead acetate paper." This reaction is used in qualitative inorganic analysis. It is a semiconducting material with niche uses.įormation, basic properties, related materials Īddition of hydrogen sulfide or sulfide salts to a solution containing a lead salt, such as PbCl 2, gives a black precipitate of lead sulfide. Galena is the principal ore and the most important compound of lead.
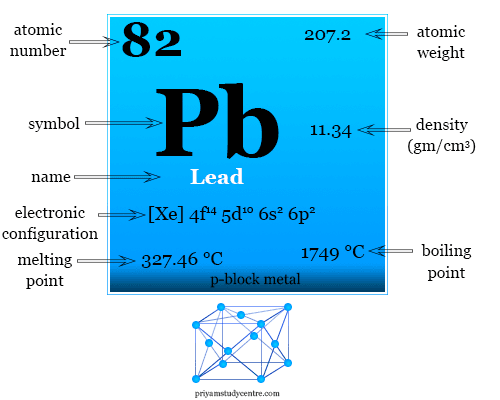
Lead(II) sulfide (also spelled sulphide) is an inorganic compound with the formula Pb S.


 0 kommentar(er)
0 kommentar(er)
